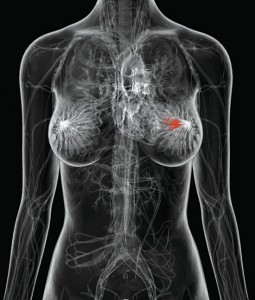
 Over 1.1 million women are diagnosed worldwide each year with breast cancer,1 and this number has almost doubled since 1975.2 In addition, breast cancer is the most prevalent cancer in the world, with 4.4 million survivors up to 5 years following diagnosis,1 and millions more surviving longer than 5 years.
Over 1.1 million women are diagnosed worldwide each year with breast cancer,1 and this number has almost doubled since 1975.2 In addition, breast cancer is the most prevalent cancer in the world, with 4.4 million survivors up to 5 years following diagnosis,1 and millions more surviving longer than 5 years.
In the United States, the American Cancer Society reports that approximately 250,000 women are diagnosed each year with breast cancer.3 And while 40,000 women die annually in this country from breast cancer, there are, at any point in time, an estimated 2.3 million breast cancer survivors in the U.S.3
Although the overall incidence of breast cancer has been increasing for more than two decades, there has been a gradual reduction in mortality beginning in 1990 when the rate began to decline by 2.3% annually.3 This improvement in survival has been attributed to two factors, roughly equal in effect: 1) early detection through mammography screening programs, and 2) the increased utilization of adjuvant systemic therapies (antihormonal drugs and chemotherapy).4 Improving the former – the impact of early detection – is the focus herein.
Assessing the Impact of Early Detection Through Screening Mammography
Although early diagnosis has been a pervasive theme for improving survival rates with many types of cancer, the scientific evidence that mass screening improves survival is remarkably sparse. Even today, there is no evidence to support mass screening for common cancers such as lung5 and prostate6 in spite of available testing for early detection. Undaunted, health care professionals and the public have consistently demonstrated enthusiasm for cancer screening,7 and it is still possible that screening studies underway will validate early diagnosis as a principle to be applied to most types of cancer.
Currently, however, breast cancer is one of the few types of malignancy where mass screening has already been validated as reducing mortality, in this case through the use of mammography. (Self-exam, although recommended in all guidelines, remains controversial as to its impact on mortality reduction.) No method of cancer screening has been studied with such scrutiny for so many years, as has mammography; yet, the proof of mortality reduction did not come easy.
In fact, at the same time that the clinical trials for screening mammography were being launched, a new theory of breast cancer biology emerged indicating that breast cancer was a systemic disease at its inception, implying that early diagnosis would have little or no impact. This so-called Fisher theory8 of breast cancer biology was supported by findings in the clinical trials that confirmed the equivalency of breast conservation (lumpectomy) to mastectomy.9 Yet, after multiple mammography screening studies and many years of controversy, it is now generally accepted that mammography reduces breast cancer mortality in screened populations.10 Thus, the concept of breast cancer being systemic at its inception had to be modified in order to explain how early detection could make such an impact. This resulted in the Spectrum Theory11 that dominates today, a theory which suggests a spectrum of biologies such that many, but not all, breast cancers are vulnerable to early detection, supporting efforts to improve mammography as well as other diagnostic measures.
The benefit of screening mammography has now been reflected in mortality reductions seen outside the confines of clinical trials in countries where mammography is standard screening practice.12 Trying to quantify the benefit, though, is a challenge. Certainly, the benefit of screening only applies to women who comply with guidelines. Even then, however, some patients adhering closely to screening guidelines will die from breast cancer.
For instance, when prospective randomized studies indicate a “30% reduction in breast cancer mortality” for women in mammography screening programs, this is based on a comparison to the mortality rate demonstrated in a control group where women did not have regular mammograms. If such a study included, for instance, 10,000 patients, and of those in the control group who developed breast cancer, 100 women died of their disease, then a “30% reduction” means that in the mammography screening group, only 70 women died from breast cancer. The obvious question is, “Why did the 70 women who were compliant with mammography still die?”
This brings us back to the issue of breast cancer biology. Perhaps, these deaths occur in women with more aggressive breast cancers in whom early diagnosis makes no difference. However, biology is not the only determinant regarding the efficacy of cancer screening. The other powerful factor is the sensitivity of the screening tool. Stated alternatively as a question: What percentage of detectable breast cancers are actually detected by mammography? Although it is often stated as a truism that breast cancer is present 5-10 years prior to detection by mammography, this concept is loaded with caveats and, even if valid, does not become clinically relevant unless the cancer is large enough to be detected through current technology, thus allowing therapeutic intervention. Thus, the question of mammographic sensitivity relates to occult cancers that are missed, yet clinically detectable and treatable.
Sensitivity of Mammography
While the controversies about breast cancer screening lasted for decades and focused almost entirely on mortality reduction in the prospective, randomized mammography screening trials, a critical parameter went virtually unnoticed in both the radiologic community and physicians in general – mammographic sensitivity.
Popular media continue to propagate the myth that mammography can find “90% of breast cancers in women who have no symptoms of the disease.” 13 Even the American Cancer Society makes the position statement that “mammography will detect about 80-90%” of asymptomatic cancers,14 though the origin of this sensitivity level is not referenced. In fact, the origin of the “mythical 90%” is difficult to find, perhaps derived from those early studies of mammography that measured sensitivity of the new tool in women with palpable cancers. If cancers are palpable, of course, this is no longer “screening asymptomatic patients,” yet it appears that the medical community then applied this “90% sensitivity” to non-palpable cancers as well (at the time, there were no other proven modalities to detect breast cancer).
While certain subsets of patients, based on advanced age or low density mammograms, will demonstrate 90% sensitivity with mammography, there is no support in the published literature for such an optimistic value when addressing the general screening population. Mammographic sensitivity is, in fact, highly variable among individuals and should be a customized number for the informed patient; however, for general population screening, mammographic sensitivity doesn’t come close to 90%.
In a rare review of the historical mammography screening trials,15 sensitivity for mammograms alone ranged from 39% in the Health Insurance Plan to the highest figure of 92% in a subset (ages 70-74 in the Swedish Two-County study); however, most sensitivity determinations were in the range of 60-66% overall, with Malmo at 61%, Edinburgh 63%, CNBSS-1 (ages 40-49) 61%, and CNBSS-2 (ages 50-59) 66%.
These low sensitivity values have been attributed to the fact that these trials began three decades ago and that mammographic quality has been vastly improved in the years since. However, using the most advanced technology in the recent Digital Mammographic Imaging Screening Trial (DMIST) coordinated by the American College of Radiology,16 the overall sensitivity as defined by a 12-month follow-up period revealed only 70% sensitivity with digital technology and 66% for state-of-the-art film screen technology, comparable to the sensitivities in the historical trials.
But these remarkably low sensitivity values may, in fact, be optimistic. One of the many problems plaguing the quantification of mammographic sensitivity has been the definition of a “missed cancer.” How does one discover an undiscovered cancer? And how does one define such a discovery? Historically, the accepted definition comes through long-term follow-up data, with any cancer discovered within 12 months following a negative mammogram considered to be a “missed cancer” on the previous study.
Nothing is magical about 12 months of follow-up, of course, and in the DMIST study noted above,16 investigators took the unconventional approach of calculating sensitivity based on 15-month follow-up as well, under the assumption that a cancer discovered within a 15-month time frame following a negative mammogram was likely to have been present on the prior study. Remarkably, mammographic sensitivity under this definition turned out to be 41%, and the difference between digital technology and film screen disappeared entirely.
It has to be considered that long-term follow-up is a harsh definition, given the fact that aggressive interval cancers (those cancers with rapid doubling times that emerge between screening studies on self exam or clinical exam) may not have been clinically detectable on the prior study. Also, cancers are sometimes detectable on the prior mammogram, but are missed due to radiologist error; or, changes on X-ray might have been so subtle that the threshold for biopsy was not met. These issues point out the difficulty in trying to assess true mammographic sensitivity using the historical standard of long-term follow-up. More recently, with the introduction of breast ultrasound and breast MRI, the ability to determine true mammographic sensitivity through multi-modality imaging is vastly improved. As it turns out, the 70% sensitivity using the standard definition in the DMIST study may be too high.
Mammographic Sensitivity as Defined by Breast MRI
Multiple prospective trials have now addressed multi-modality imaging for the detection of breast cancer in high-risk women. While breast ultrasound can routinely identify breast cancers missed by mammography, especially in women with dense breasts, its sensitivity is overshadowed by MRI. Thus, breast MRI has recently been recommended as an adjunct to mammography in the new American Cancer Society screening guidelines for high-risk women that emerged from the prospective multi-modality studies.17
In spite of many differences among the prospective, non-randomized trials of multi-modality imaging in asymptomatic women, a consistent feature is noted in that MRI demonstrates twice the sensitivity of mammography. In a recent analysis combining the five largest studies, the sensitivity of mammography alone was 40% while the sensitivity of breast MRI alone was 81%.18 It is largely because of this large improvement in sensitivity in all studies that the American Cancer Society has endorsed breast MRI for screening even in the absence of corresponding mortality reduction data. It is noteworthy that the only way to achieve the oft-quoted “90%” sensitivity in these studies was to perform both mammography and breast MRI. Mammograms continue to be valuable in the detection of microcalcifications (not well seen on MRI), which can be the earliest indicator of malignancy. Then, MRI can detect the non-calcifying cancers that are lost in the background density of the normal breast tissue on X-ray.
A general misconception exists, again in the popular media, that these prospective MRI trials validated the use of MRI only for women with mutations in one of the BRCA genes. In fact, only one of seven trials was limited to BRCA-positive patients,19 and it was possible to enter one of the trials when lifetime risk of breast cancer was only 15%,20 barely above the risk seen in the general population. Overall, family histories predominated as the entry criteria in these trials, and this is reflected in the new American Cancer Society guidelines, which recommend using mathematical models that focus on family history to identify patients at “20-25% lifetime risk” for the development of breast cancer. For these women, the addition of annual breast MRI to annual mammography is recommended. 17
In addressing the poor mammographic sensitivity in these trials, it is often pointed out that all published studies have been limited to high-risk women, and that these trials are skewed toward younger women with higher-density mammograms. The implication here is that the sensitivity of mammography in the general population is better; however, it is interesting to note that the 40% sensitivity for mammography in high-risk patients in these MRI trials is virtually identical to the 41% sensitivity for mammography in the general population in the DMIST trial when using the 15-month follow-up definition.
In addition, subset analysis in the aforementioned Toronto study,19 where all the participants were BRCA-positive, revealed that the difference in sensitivity between MRI and mammography was no greater for women under 50 than for women ages 50 and older.21 Then, another subset analysis by the same researchers revealed that even in the low-density group, the sensitivity of mammography was less than 50%.22 The findings in both of these subset analyses were contrary to the expectations of the investigators,23 and it emphasizes the importance of close scrutiny in these trials before attributing the poor performance of mammography to a skewed study population.
Regardless of the measure used, mammographic sensitivity does not come close to the “90%” figure that is so prevalent, still conspicuous today as a disclaimer found at the bottom of many radiology reports. At best, using the more lenient definition of the DMIST study, mammographic sensitivity is 70% while it may be as low as 40% overall as evidenced by the high-risk multi-modality studies or, alternatively, the 15-month definition of the DMIST study. For individuals, the range of sensitivity with mammography extends from near-zero in patients with extreme density to near-100% in women with complete fatty replacement. But for the general population, one way to consider the sensitivity of mammography is as follows: For every mammographic discovery of a breast cancer in a screening program, there is another asymptomatic woman sent out the door with a detectable breast cancer and a “negative” mammogram report.
Upcoming Improvements in Mammographic Sensitivity
To date, minimal improvement in mammographic sensitivity has come through technologic advances. Certainly, the quality of the images today is far superior to what was seen decades ago, but if a cancer, being white on X-ray, is buried in the comparable “whiteness” of dense breast tissue, it is likely to be invisible no matter how sharp the images.
Witness the minimal improvement in cancer detection seen with the advent of digital mammography as noted in the DMIST study above,16 and the continued controversy as to whether or not CAD (computer-assisted diagnosis) improves sensitivity, or if it is helpful only for less experienced radiologists.24
The great weakness of mammography is its dependence on anatomic contrasts, when, in dense breast tissue, such contrasts may be absent. Thus, there is an inverse correlation between mammographic sensitivity and mammographic density. MRI is impacted only slightly by breast density, as it relies on the physiologic identification of cancers using a contrast-enhancing agent (gadolinium) that is infused as part of the procedure, not to mention the very thin slices (1-2 mm) of the images. Similar to the principles of MRI, upcoming improvements in mammography include using intravenous infusions as part of contrast enhanced mammography,25 as well as tomosynthesis26 wherein the images of the breast are made in slices (albeit much thicker than MRI). Preliminary evidence suggests there will be improvements in mammographic sensitivity, and perhaps the greatest improvement will come when both contrast-enhancement and tomosynthesis are used together. However, many limitations with mammographic screening will continue, most notably the extraordinary costs for this added technology when, in fact, it is critical for mass screening programs to keep costs controlled.
Persistent Limitations with Screening Mammography in Spite of Improvements
The purpose of screening is to save lives, not money. Many are surprised to learn that screening mammography as a public health policy does not decrease health care expenditures. It adds. While it may be intuitive to think otherwise, it was well known even in the days of sub-$100 mammograms that asymptomatic screening adds to the cost of finding and treating breast cancer. Even with a disease as common as breast cancer, the vast majority of women who undergo mammography do not have cancer, yet mammographic screening prompts false-positives with the attendant call-backs for special views, additional ultrasound studies, diagnostic MRI, image-guided biopsies, and surgical biopsies.
And, simply finding a cancer on mammography does not translate into a “saved life.” Some cancers will be cured even if discovered later on exam, while other cancers will already have metastasized when discovered on mammography. In fact, one estimate by a noted mammography advocate/radiologist is that a single life is saved for every 7.4 breast cancers detected by mammography, requiring 1,460 mammographic examinations.27 Opponents of screening mammography would likely quote statistics even more inefficient.
Much of the cost-effectiveness literature is therefore based on parameters that acknowledge the fact that screening “costs” rather than “saves,” with a typical example being “marginal cost per year of life saved” or MCYLS (note: this is “life saved,” not cost savings). Thus, when one study,28 for example, claims that screening all women from ages 40 to 79 is “cost effective” because the MCYLS of $18,800 is comparable to less frequent imaging after age 50 where MCYLS is $16,100, we are learning that “cost effectiveness” is a relative term used for comparisons, as well as deeming various approaches “acceptable.” Yet, these are costs just the same. Remembering that MCYLS is a “per year” value, one can readily calculate the cost of saving the life of one 40-year-old woman to be well in excess of a half million dollars given her normal life expectancy.
And when computer-assisted detection (CAD) is added to mammography, we find a study revealing that the MCYLS is 19% greater, again within the “acceptable” range of cost-effectiveness.29 But what will be the additional increases in MCYLS when tomosynthesis becomes part of routine screening? And when contrast-enhanced mammography is added? With gradual cost elevations, we can easily lose sight of the fact that asymptomatic screening is extraordinarily expensive. As we enter this era of explosive growth in technology with regard to breast imaging, we have to consider the possibility that we could greatly exceed our resources for asymptomatic screening, and that sophisticated imaging might best be considered as the second line of defense for localization of disease, subsequent to a low-cost general screen.
But rising costs are not the only limitation of mammography. Compliance has been variously reported using different criteria, with estimates greater than 50% since 19903 when simply having a mammogram within the past 2 years is considered “compliant.” However, when stricter criteria are utilized, more likely in line with utilization that translates to saved lives, one study revealed a mere 6% of women who received a mammogram in 1992 were compliant with annual mammograms over the next 10 years.30 Multiple reasons have been identified for the lack of higher compliance, but fear of radiation exposure, cost, pain with breast compression, and general fear of negative outcomes are among the many listed reasons.
Then, additional limitations in mammography exist in that women under the age of 40 are not advised to undergo mammograms unless risk factors are present. Thus, an estimated 11,000 women in the U.S. who will develop breast cancer each year prior to the age of 403 are disenfranchised from mammographic screening. The situation is far worse in many countries where access to mammograms are limited or completely non-existent. Clearly, the benefits of mammographic screening are limited by geography, age, economics, social, and psychological issues.
Mortality Reduction Re-visited in Light of Poor Mammographic Sensitivity
Simply because a screening tool can detect cancer does not automatically translate to a reduction in mortality. As noted above, breast cancer screening with mammography is actually the exception rather than the rule, as a mortality reduction has indeed been confirmed with mammographic screening as opposed to most other cancers where the evidence is still lacking for screening efficacy.
Proving a mortality reduction can only be accomplished through prospective randomized trials, where one group is screened and the other group is not screened. This approach is mandatory to nullify powerful biases that otherwise cloud non-randomized study results: lead time bias, length time bias, overdiagnosis bias, and selection bias. With these biases negated through prospective randomization, only two variables remain that determine efficacy: 1) the natural history (biology) of the disease as it pertains to its interruption through diagnosis/treatment, and 2) the sensitivity of the screening tool. Issues such as disease prevalence and incidence, patient compliance, and the specificity of the screening tool, pertain more to the socioeconomic realities of screening, rather than effectiveness as defined by a reduction in mortality.
The importance of the mammographic screening trials that proved a reduction in mortality cannot be overstated, not necessarily as a testimony to X-ray technology, but rather in demonstrating the validity of early detection as a principle in the fight against breast cancer. The more recent realization that this effective early detection was accomplished with a tool that has only modest sensitivity opens a door to huge potential. Simply stated, if a tool with only 40 to 70% sensitivity can reduce breast cancer mortality, then not only is early detection valid, but it is also a more powerful approach than ever imagined. What might be accomplished with an approach that has 80% sensitivity? Or 90% sensitivity?
When it comes to breast MRI, critics have charged that we need the same prospective, randomized trials to prove a reduction in mortality. But that may not be the case at all. In the mammography trials, where it was unknown if early detection was valid or not from a biologic standpoint, such trials were mandatory. Now that the concept of early detection has been validated, the only remaining variable is the sensitivity of the screening tool. The natural history of breast cancer and the epidemiologic biases have already been accounted for.
It is reasonable to theorize that a doubling of sensitivity as seen with breast MRI will result in a doubling of the reduction in breast cancer mortality. Whether or not finding cancers “earlier” than mammography with MRI has a measurable benefit is unknown; however, the obvious benefit is simply finding the “other 50%” of detectable cancers being missed by mammograms, those tumors large enough to be seen by mammography, but are simply hidden by dense breast tissue.
Clearly, the American Cancer Society did not wait for a mortality reduction with breast MRI before endorsing its use. The sensitivity data alone was so powerful with MRI, along with a dramatic plunge in the incidence of interval cancers, that this highly sensitive modality is now part of the screening regimen for high-risk women.
Although it is impractical to consider breast MRI screening for the general population, to the point that the American Cancer Society actually advises against this practice, there is no reason to believe that the wide disparity in sensitivity between mammography and breast MRI will be any different for the average risk patient. A woman without known risk factors is facing a 13% lifetime risk for breast cancer,3 a figure that is already high, and not that much different than so-called high-risk patients who qualify for MRI screening at 20% lifetime risk, especially when considering that the risk differential is spread out over a 40-year period, or an increased risk of less than 2/10ths of 1% per year.
The hesitancy to recommend widespread screening with MRI is based not only on the limitations imposed by the available published data being limited to high-risk women, but also on the lack of breast MRI expertise, the lack of available facilities, and staggering cost implications with MRI costing 5 to 10 times as much as a mammogram.
In short, we have all the ingredients for creating a massive reduction in breast cancer mortality: 1) the impact of early detection and interrupting the natural history of breast cancer has been proven, 2) the epidemiologic biases that interfere with this proof have been controlled through prospective, randomized trials, and 3) a greater than 90% sensitivity can be accomplished by combining mammography and breast MRI. Yet, this is where the aforementioned socio-economic realities of screening come into play. Given the false-positives of mammography and MRI, the overall costs of dual-modality screening, disease prevalence and incidence, and patient compliance, the prospects of screening the general population with both mammography and MRI are dim. In an era of health care cost containment, one cannot reasonably propose multi-modality screening for the general population. Thus, most investigators have been focused on limiting multi-modality screening to high-risk patients.
Strategies to Improve Screening Efficiency Based on Risk Assessment and BRCA Genetic Testing
The profound improvement in breast cancer detection with breast MRI is countered by the socioeconomic realities noted above. Thus, clinical trials using breast MRI focused exclusively on high-risk patients in an effort to have the highest possible yields that would allow reasonable cost-effectiveness. To date, cost-effectiveness studies (usually rather crude measures based on thought experiments) have been limited to breast MRI applied only to BRCA-mutation carriers,31 and there is little information regarding the costs for other high-risk groups. But with MRI costing 5 to 10 times that of mammography, there is little doubt that screening women at lower levels of risk than gene-carriers is going to have enormous financial consequences.
Women who test positive for the BRCA genes were originally considered to be at an 85% lifetime risk for the development of breast cancer, though these estimates were derived from families with a high degree of penetrance; so, for women unselected for family history, the lifetime risk appears to be 65% for BRCA1 mutation carriers and 45% for BRCA-2.32 These lower values are still remarkably elevated above baseline risk and a sharp contrast to women at a 20% lifetime risk who are also called “high-risk” in the ACS screening guidelines. Still, the point of focusing on these patients in the MRI screening studies was to highlight yields in the context of cost-effectiveness.
All prospective clinical trials with breast MRI to date have utilized a positive family history for the entry criteria, thus forming the basis of the new American Cancer Society recommendations for MRI screening. However, this has been followed by circular reasoning that suggests MRI is effective only in these high-risk populations. In fact, the American Cancer Society has specifically advised against screening in the so-called “normal risk” population. And this is where the lines have blurred between cost-effectiveness and straightforward effectiveness.
There is no biologic or radiologic reason to suggest that MRI only works in the high-risk population; in fact, it would be ludicrous to suggest this as the case. The high sensitivity of MRI has been demonstrated in many non-screening studies that address its usefulness in diagnostic situations and pre-operative staging for newly diagnosed patients. MRI should have twice the sensitivity of mammography in all situations, no matter what its indication and no matter what the lifetime risk of the patient may be.
In fact, one of the prospective screening trials for MRI33 reported sensitivity as a function of risk level. In this study, only 8% of the participants were BRCA-positive (n=43), and for this group, mammographic sensitivity was 25% (2 of 8 cancers discovered by mammography) and MRI sensitivity was 100% (8/8). However, similar differences were seen at all levels of risk. Patients at a 21-40% lifetime risk (n=241) had a mammographic sensitivity of 25% (5/20) and a MRI sensitivity of 100% (20/20). Then, in the lowest risk group where women had only a 20% lifetime risk (n=110), as compared to the general population risk of 13%, mammographic sensitivity was 50% (3/6), while MRI sensitivity was again 100% (6/6). These results support the common sense concept that improved cancer detection with MRI is not a function of risk levels, but due to the inherent attribute of the screening tool.
Nevertheless, using a variety of mathematical models to establish lifetime risk, or through BRCA genetic testing, are the approved approaches today for selecting patients for high-risk screening with MRI. Yet, the inherent weakness of this approach has to be considered – whenever levels of risk are utilized as the sole criteria, the majority of women who will develop breast cancer are excluded. And, the higher the risk level required for MRI screening, the greater the number of women that will be excluded. This is a simple function of the fact that only the minority of women who develop breast cancer have a positive family history for the disease.
The American Cancer Society guidelines acknowledge the need for “more research” when it comes to other risk factors such as atypical hyperplasia, a prior history of breast cancer, or breast density, addressing the population where lifetime risk is between 15 and 20%. The lower end of this controversial group (at 15% lifetime risk) is virtually identical to the 13% lifetime risk of the general population, and becomes a meaningless difference when one considers that these risks are spread out over decades. Thus, many problems exist in the attempts to justify current guidelines for MRI screening that are remarkably aggressive for women above 20% (annual MRI in addition to annual mammography, beginning at age 30), while all others are to simply begin mammography alone at age 40.
Risk is, in fact, only half the story. Theoretically, lifetime risk is intended to translate into the probability that a MRI study, or sequential MRIs, will be positive. But there is another factor present that may be as important as risk, and this is the probability that a cancer will be missed on mammography. And the primary determinant as to whether or not a cancer will be missed is the degree of breast density on mammography. In the American Cancer Society guidelines, breast density is singled out as one of the risk factors that requires “more research.” But it is unclear whether or not the ACS is referring to breast density as a risk factor (where it has unappreciated power), or whether it is referring to its influence on mammographic invisibility. Nevertheless, the case has been made that if one used breast density alone to determine the appropriateness of adding MRI to mammograms, more occult cancers would be discovered in the screening population than by relying on risk.34
In summary, centers of excellence have adopted an approach for utilizing screening breast MRI that rely on complex mathematical models and/or genetic testing that are predicting the likelihood of future disease, but only slightly impact the likelihood of disease at the time of the MRI study. Breast density would, perhaps, yield more cancers discovered through MRI by focusing on those women where mammography is at its worst. And the most efficient approach, in both yield and cost-effectiveness, would likely be a combination of lifetime risk and breast density.
But what would be ideal, going beyond risk and beyond density, would be if a screening tool – a blood test – could identify patients with mammographically occult breast cancer, such that the probability of MRI discovery would be dramatically heightened. Such an approach would address the current risk of occult disease as distinct from the future probability that a disease might occur.
Blood Testing as a Screening Tool
Currently, if a 30 year-old woman has been calculated to be at a 40% lifetime risk for breast cancer, a level where general agreement would dictate the need for MRI screening, she has nearly a 1% probability that a cancer will develop each year. Using the assumption that this cancer will be missed on mammography due to high density, there is a 1% chance that a single screen with MRI will identify a cancer. In fact, this is a respectable yield, given our acceptance of comparable yields with screening mammography in the general population. Thus, 100 women at comparable risk would need to be studied with MRI to find one cancer. However, the screening cost to find this one cancer will approximate $100,000 for the 100 MRIs, not to mention the extra costs related to call-back studies and false-positive biopsies. Then, remembering that only 1 in 7 image-discovered cancers actually results in a life saved, it is likely that costs will prove to be several million dollars to save one life with breast MRI screening – and this is when MRI is used selectively, at risk levels far above the minimum risk elevation suggested by the American Cancer Society guidelines.
Needless to say, if that one individual could be singled out from the 100 as having occult breast cancer through a blood test, then MRI could be recommended and the cancer discovered with remarkable efficiency. Such a test would be highly valuable if it simply reduced the number of women that had to be screened with MRI from 100 down to 5 women. Even reducing the number down to 10 or 20 would be a major advance with regard to efficient utilization of breast MRI.
Applied thusly, the standard for breast screening would become annual mammography and annual blood testing. If mammograms were negative, but blood test positive, the patient would then undergo diagnostic MRI for confirmation and localization of the cancer. Such an approach would revolutionize breast cancer screening, making the potential for MRI available to all women, with no exclusions based on “normal risk.” Remembering that mammography is only identifying approximately one-half of detectable cancers, this approach would, in theory, double the number of early detections, and thus double the mortality reduction seen with mammography alone.
Other benefits of a screening blood test would be the utilization in women considered “too young” for mammography, the disenfranchised 11,000 women each year who develop breast cancer prior to the age of 40.3 Annual blood testing could begin at age 25 without breast imaging of any type, then if the blood test is positive, young women could undergo any combination of ultrasound, MRI, or mammography for disease confirmation and localization. Similarly, older women who currently refuse mammography could undergo blood testing as a single screening tool, with the understanding that they would consent to breast imaging if positive.
Lastly, such blood testing could be considered as the sole modality for those countries where breast imaging equipment is sparse or non-existent. The impact on mortality reduction through blood testing in these locations would vastly exceed what can be anticipated in those countries accustomed to mammographic screening where a modest reduction in mortality is already being accomplished.
What would the sensitivity of screening blood test need to be for breast cancer? When this concept is introduced, it is easy to make the analogy to PSA blood screening for prostate cancer, and this is a valid comparison to explain the rationale. But surprisingly, the sensitivity of PSA is controversial, largely because the natural history of prostate cancer discovered by PSA testing is unknown. And, the impact of PSA screening is also unknown since a mortality reduction through early detection has not yet been demonstrated. So, while the comparison is fair for illustrative purposes, there is no measure available regarding an acceptable sensitivity for a cancer-screening blood test.
On the other hand, for breast cancer, we do have a non-blood-based measure by comparing to the sensitivity already provided by mammography. For those radiologists and clinicians who believe that mammograms alone are providing 90% sensitivity, a blood test will make little sense. But for those who realize it takes both mammography and MRI to achieve such a high sensitivity, and that mammograms alone are only 40%-70% sensitive (depending on the definition), the benefits of a blood test become readily apparent.
Even if a blood test only has sensitivity equivalent to mammography, it is likely that such a test will be helpful, as it may be picking up a “different” 40-70%. There should be no variance in sensitivity based on breast density with a blood test, as occurs with mammography. A blood test would be “blind” to breast density, so it is likely that even modest sensitivity would identify cancers missed by mammography. Certainly, though, the greater the sensitivity of a blood test, the more useful it will be. And while 90% sensitivity and 90% specificity is always a nice goal, sensitivity levels below 90%, perhaps below 80%, could still revolutionize breast cancer screening when it is realized that the “gold standard” of mammography has lowered breast cancer mortality in spite of its remarkably low sensitivity.
The caveat here is actually “specificity” because, when it comes to screening, false-positives cause considerable problems. What happens after a positive blood test, but a negative MRI? Certainly, short-interval follow-ups with MRIs would be recommended, but for how long? And, what is going to be the impact on patients wherein considerable anxiety will be transmitted to women with false-positive results?
False-positives with a blood test would also add to the burden of false-positives already being seen in breast imaging where such results are an inherent part of mammography, ultrasound, and MRI. And when screening large numbers of women, a relatively low false-positive rate can still translate into a large number of patients who must deal with the uncertainties imparted, including the realization that an alleged false-positive might actually be pre-dating the clinical appearance of a breast cancer. It is conceivable that 5 to 7 years of aggressive follow-up might be needed to confirm that a positive result is, indeed, false.
Although this scenario is problematic, if it turns out that a blood test is actually pre-dating the clinical appearance of cancer, then such patients might be ideal candidates for the antihormonal measures that are currently FDA-approved to prevent breast cancer. For instance, if 20% of women who are called “false-positive” end up getting breast cancer over the next 5 years following blood testing, when only 1% would have been anticipated, the blood test would clearly be identifying the development of breast cancer well before MRI. And such subclinical disease could prove susceptible to the anti-hormonal agents that are known to prevent the emergence of clinical breast cancer.
Although this “earlier than MRI detection” would introduce a new set of controversies – as to whether or not MRI follow-up would still provide detection early enough, or if antihormonal drugs are indicated for suppression of emerging disease, or if some women might leap to preventive mastectomy – such information would still be of great help in the long run after the necessary clinical information emerged to guide patient options.
In summary, given the ever-present balance between sensitivity and specificity, when it comes to screening, the focus will be on specificity, while only a modest sensitivity would still be helpful in light of the relatively poor sensitivity of mammography.
Blood Testing as a Diagnostic Tool
Up to this point, the foregoing discussion has focused entirely on screening, first with the weakness of mammography, then the vast improvement but inefficiency of MRI, and finally on a blood test that would efficiently select patients for MRI. However, given a blood test that can detect early cancer, such a test would also be useful in the diagnostic setting for the radiologist.
In spite of the BI-RADS® system for breast imaging interpretations as outlined by the American College of Radiology,35 which guides radiologists in their decision as to whether or not to perform a biopsy, the interpreter of breast images is often faced with ambiguous findings wherein the decision for biopsy is subjective. This invites circular reasoning in the interpretation, wherein the radiologist decides not to biopsy, and thus issues a “BI-RADS 3” level on the official report to support the position. Or, the radiologist might be uncomfortable with observation for a particular finding, and could issue a “BI-RADS 4” level on imaging to support the need for a biopsy. Yet, a different radiologist might issue different BI-RADS levels in both situations. Subjectivity cannot be eradicated in diagnostic imaging, no matter how elaborate the protocol.
The use of a blood test in the diagnostic work-up could then be helpful in either of two ways: 1) With strong sensitivity and thus strong Negative Predictive Value (NPV), a negative blood test would give the radiologist support not to perform a biopsy; or, 2) With strong specificity and thus strong Positive Predictive Value (PPV), a positive blood test would offer support to go ahead with the biopsy. Unlike screening wherein a difficult balance exists between sensitivity and specificity, a blood test with great strength in one or the other could still play a key role in diagnosis when used appropriately.
Currently, the vast majority of breast biopsies based on imaging are negative, and the BI-RADS system has had little impact in this regard. While BI-RADS 5 findings are almost always cancers and radiologists need little help here, only 15-25% of BI-RADS 4 findings will be positive, and many breast centers have positive yields lower than this. This low PPV, even after 30 years to improve mammography, is a major contributor to the inefficiency of screening, not to mention the additional patient anxiety associated with screening. A blood test that could refine the decision-making process for the radiologist would have an enormous benefit.
Many diagnostic situations arise wherein blood testing might help. It is common for findings on breast imaging to be not only ambiguous, but multiple. A breast radiologist is confronted almost daily with mammograms that are dense with multiple “probably benign” calcium clusters, various densities, and perhaps ambiguous findings on ultrasound as well. A blood test could help steer these patients toward (or away from) MRI or biopsy, depending on the specific strengths of the blood test.
Radiologists are not the only specialists who need help. Surgeons and primary care physicians often deal with findings on physical exam that are quite concerning, yet conventional breast imaging, sometimes including breast MRI, are negative. There are some cancers, most notoriously invasive lobular carcinoma, that simply do not appear on any form of breast imaging. A blood test would be helpful, once again, in determining the need to proceed with biopsy or observation. Other symptoms, such as breast pain, nipple discharge, skin changes, etc., can sometimes be the presenting complaint in breast cancer, yet imaging can be negative, so the diagnostic applications of a blood test are many.
Blood Testing as a Tool for Cancer Follow-up
A blood test that is capable of detecting early breast cancer, still contained in the breast, is likely to be an effective tool as part of cancer follow-up as well. This is especially true for women whose abnormal results pre-operatively return to normal post-operatively. A subsequent abnormal test is likely a signal of recurrence disease.
In fact, it is here that most of the blood testing research has concentrated in the past, with “serum tumor markers” in current use by many medical oncologists. CEA, CA15-3, CA27.29 have all been found helpful in the identification of recurrent cancer, but with sensitivity and specificity issues that limit usefulness, even in the metastatic setting where there is questionable benefit when preclinical lead times with positive results are only 2 to 9 months.36 None of these common serum markers has been found to be sensitive enough to use for identifying early breast cancer, however.
If there is little impact in diagnosing metastatic cancer 2 to 9 months earlier than when the disease would become obvious anyway, then a blood test for early breast cancer may not have a great deal of benefit over the currently utilized serum markers – at least, not until curative measures are developed for metastatic disease. However, for those women undergoing breast conservation, detection of recurrence within the breast is quite important as a salvage mastectomy can still result in a cure in many cases. Thus, an accurate blood test for breast-only disease would play a major role in the follow-up of breast cancer patients who have elected breast conservation as their initial locoregional treatment strategy.
History of BC-SeraPro® Blood Testing by Power3 Medical Products, Inc.
In March 2007, Power3 Medical Products received CLIA Certification (Clinical Laboratory Improvement Amendment) for the BC-SeraPro® test to be used in the early detection of breast cancer. This achievement began when Power3, focusing on proteomics, acquired a set of ductal fluid biomarkers developed at M.D. Anderson Cancer Center in Houston, prompting a multi-site clinical trial to determine if these biomarkers could aid in the diagnosis of breast cancer via nipple aspirate fluid (NAF).
The leadership for this study was provided by Essam Sheta, PhD, Director of Biochemistry and the Power3 CLIA (Clinical Laboratory Improvement Amendment) Laboratory Director. He was awarded the prestigious Fulbright Scholarship at The University of Texas at San Antonio where he provided the first bidomain structural proof of nitric oxide synthase, the most complex human enzyme known. This was followed by his unique methodology in the first commercial production of the enzyme by overexpression in E. coli. As an Associate Professor of the Department of Biochemistry at Alexandria University, he supervised several graduate students for Master and Ph.D. degrees in biochemistry and served on the Fulbright Committee Review Board in Cairo. Dr. Sheta is assisted by Ira L. Goldknopf, PhD, Director of Proteomics at Power3 Medical Products and a pioneer in the field. Dr. Goldknopf was the discoverer of a central protein system involved in cell proliferation, apoptosis, and most major cellular regulatory functions. This “ubiquitin system” was cited in the 2004 Nobel Prize for Chemistry.
While the premise of the ductal fluid test was valid, an enormous obstacle with this approach to improve breast cancer diagnosis was the difficulty encountered in the retrieval of bilateral NAF from patients through a laborious process of breast compressions. However, as part of this clinical trial, serum samples had been obtained as well, yielding a number of unique biomarkers signaling the presence of breast cancer.
This finding led to a collaborative research agreement with Mercy Women’s Center in Oklahoma City, a multidisciplinary breast screening and diagnostic facility that is part of Mercy Health Center. Mercy hospital is the busiest location in the state of Oklahoma for the diagnosis and treatment of breast cancer, and the Women’s Center is one of the most experienced sites in the nation with breast MRI. Mercy also has an active risk assessment and genetic testing program, and the director of that program, Dr. Alan Hollingsworth, had been collecting and storing serum samples on breast cancer patients and controls for a number of years.
Figure 1:
From 775 of these samples, 22 protein markers were identified (patents pending) that were differentially expressed in the serum of breast cancer patients. Using 2-D gel electrophoresis and gel image analysis (see Figure 1), the effects of individual and combined markers were used to differentiate breast cancer patients from controls (see Figures 2 & 3). When the test had evolved to the point of potential clinical utility, a blinded study was performed, with results presented at the 30th Annual San Antonio Breast Cancer Symposium held in December 2007.
Figure II:
Figure III:
Presentation at the San Antonio Breast Cancer Symposium
Using 98 samples obtained from two clinical sites – Mercy Women’s Center in Oklahoma City and Obstetrical & Gynecological Associates, PA in Houston – BC- SeraPro® was performed. Samples were obtained from 21 healthy controls, 38 women with confirmed benign breast disease, and 39 breast cancer patients at various stages. The 39 breast cancer patients had various histologies as well, with 7 patients having Stage 0 DCIS, 4 patients with invasive lobular carcinoma, and 28 patients with invasive ductal carcinoma.
For the entire group of samples, the sensitivity and specificity were both 90%, with 35 of the 39 breast cancer patients correctly identified, and 53 of 59 controls/benign disease correctly identified. (see Table I – Healthy controls and women with confirmed benign disease were combined for linear discriminant analysis in a two-way comparison.)
Table I:
Table II:
Although 98 samples were included in the formal study, additional outcomes were analyzed for 60 previously untested samples (of the original 98) that had been blinded to the researchers with regard to clinical information. For these 60 patients, sensitivity was 80% and specificity 87% (see Table II). Breakdown of the 30 cancer patients within this grouping revealed predominantly early-stage disease, with 24 patients having Stage 0 & I breast cancer, 5 patients with Stage II disease, and only 1 patient with Stage III. Later analysis was performed as to histologic grade, ER/PR positivity, and HER2/neu status, and though sub-groups were too small for statistical differences, no trends could be noted. The accuracy of BC-SeraPro® appeared to be the same across all groupings.
Prospective Clinical Trial for BC-SeraPro® Validation
Subsequent to the study of the blinded samples above, a prospective clinical trial was begun to confirm the utility of BC-SeraPro® in the clinical setting. Under an IRB-approved protocol, patients at Mercy Women’s Center in Oklahoma City who have suspicious mammograms, undergo drawing of blood samples prior to breast biopsy. In addition, samples are drawn in healthy controls from asymptomatic women who are part of the MRI screening program and who have both negative mammograms and MRI at the time of the blood draw.
Because some women, especially those with benign breast disease, end up with BC-SeraPro® values in an intermediate range, this trial is utilizing an “indeterminate” category in order to offer helpful clinical information, minimizing the confusion caused by false-positives and false-negatives. A “positive” result is thus intended to indicate the presence of breast cancer, a “negative” result is intended to denote the absence of cancer, and an “indeterminate” result prompts the need for follow-up blood testing to see if there is a worsening proteomic pattern over time.
This approach is analogous to the system currently in widespread clinical use for OncotypeDX, a genomic-based study of breast cancers where an attempt is made to predict the likelihood of systemic cancer recurrence. Breast cancers submitted for OncotypeDX analysis are given a “low” recurrence score, which indicates no need for adjuvant systemic chemotherapy, or a “high recurrence score,” which indicates the need for adjuvant chemotherapy. However, a “mid-range recurrence score” is an open-ended result where clinical judgment is utilized, while a prospective, randomized clinical trial is in progress (TAILORx) to determine whether or not chemotherapy should be utilized for this group.
In the same fashion, Power3 plans the “indeterminate” score for BC-SeraPro® to be modified with additional experience, hoping to minimize this category over time. The balance between sensitivity and specificity is always a challenge, since gains in one parameter almost always impart losses in the other. Setting a single score for dichotomous reporting of positivity vs. negativity would limit clinical use at this early point in the clinical introduction of a new modality. Thus, the “indeterminate” category is planned for the initial launch and will likely continue as part of the assay until the database is so deep that the category can be used infrequently or dropped entirely.
The intended use for the blood test is as stated above – screening, diagnostic work-ups, and cancer follow-up. To date, however, testing has been limited to this pre-clinical prospective, blinded trial in order to provide meaningful sensitivity and specificity data for clinicians.
Competition
While it is not believed that there is another breast cancer blood test at the threshold for commercial launch, research in this area is ongoing at major biotech and pharmaceutical companies as well as smaller biotech companies using a variety of approaches such as SELDI-TOF and mass spectroscopy. The complexity and heterogeneity of breast cancer has made the development of such a test quite difficult. Unlike prostate cancer where a single antigen – prostate specific antigen (PSA) – is manufactured almost exclusively by the prostate gland, and to an elevated degree by most prostate cancer cells, breast cancer has no known common or unique antigen. Use of other solitary markers for breast cancer, such as the nuclear matrix protein NMP-66 (Matritech, Inc.) proved unsuccessful, leaving the vast majority of research focused on the identification of multiple markers followed by the integration of these markers into meaningful patterns.
Future Directions
Breast cancer is not unique in its complexity with regard to blood testing research. It appears, in fact, that most cancers under study will require an array of markers in order to arrive at a clinically useful test. For instance, the currently available CA-125 blood test for ovarian cancer has been in use for over 20 years, but is recommended sparingly for screening due to its modest accuracy. Thus, several investigators are using a multiple marker approach for ovarian cancer to replace the CA-125. The “simplicity” of tests like PSA for prostate cancer is unlikely to be found in most cancer types, so it is anticipated that the multiple marker approach will become the standard.
At the same time, imaging technology has progressed to the point where virtually any malignancy in the body can be identified by the time it reaches 1.0cm in size, using MRI, CT, PET scanning, endoscopy, as well as various combinations of these modalities. However, for the same principles as noted earlier in this document, the more complex and expensive these procedures become, the more inaccessible is their use for general population screening. The sequence of a low-cost screening blood test will very likely be the standard eventually for cancer of all types, reserving the expensive diagnostic procedures as a second-line approach for diagnostic confirmation as well as localization of the cancer to allow treatment.
The more futuristic step will be linking therapeutics to the blood test, such that abnormalities in the body might be treated according the proteomic or genomic profile of the individual cancer. Furthermore, it is not unreasonable to predict that the next generation of scientists will be able to intimately link blood testing to both cancer detection and therapy, such that all is accomplished as a single step. To assist in this goal, blood samples from patients with all types of cancer are being collected to allow biomarker discoveries that will apply broadly in the field of oncology.
The development of BC-SeraPro® is the first step toward this goal in breast cancer. After the prospective blinded trial currently underway, the blood test will need to be implemented into actual clinical scenarios at multiple sites and data collected through a registry and formal clinical trials in order to allow its role to be defined in screening, diagnosis, and cancer follow-up.
At this point, it is currently unknown where, in the clinical “life” of a breast cancer, BC-SeraPro® is detecting the malignancy – before mammography?…at the same time as MRI?…or, before MRI? The distinction may not be necessary given that the primary purpose of the blood test is to detect cancers being missed by mammography. However, the most challenging development will occur if BC-SeraPro® is actually detecting cancer earlier than MRI. This would result in the unusual situation in which clinical imaging would need to “catch up” to blood testing. Such advancements are in progress as breast MRI technology continues to improve with thinner and thinner slices (currently at 1mm with some units), as well as improved accuracy through the addition of MRI spectroscopy.37 PET mammography is also making an entry into the clinical arena, bringing with it the possibility of receptor-targeted therapies in addition to improved breast cancer detection.38
The early diagnosis of breast cancer appears to be on the eve of a revolution – a new approach to cancer detection through blood testing, first as an adjunct to mammography, indicating the need for further study, such as MRI. Then, over time, if adequate sensitivity can be reached, blood testing alone could serve as the initial sole screening methodology, not only for breast cancer but also other types of cancer, guiding the efficient use of expensive imaging technologies for disease confirmation, localization, and treatment.
1 Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74-108.
2 Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62:163-182.
3 American Cancer Society. Breast Cancer Facts and Figures 2005-2006. Atlanta:American Cancer Society, Inc.
4 Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353:1784-1792.
5 Black WC. Computed tomography screening for lung cancer: review of screening principles and update on current status. Cancer 2007; 110:2370-2384.
6 Concato J, Wells CK, Horwitz RI, et al. The effectiveness of screening for prostate cancer: a nested case-control study. Arch Intern Med 2006; 166:38-43.
7 Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA 2004; 291:71-78.
8 Fisher B, Montague E, Redmond C, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. Cancer 1977; 39:2827-2839.
9 Fisher B, Anderson S. Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347:1233-1241.
10 Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: Update 2003. CA Cancer J Clin 2003; 53:141-169.
11 Hellman S. Karnofsky memorial lecture: Natural history of small breast cancers. J Clin Oncol 1994; 12:2229-2234.
12 Feig SA. Effect of service screening mammography on population mortality from breast carcinoma. Cancer 2002; 95:451-457.
13 Park A. Breast-Cancer Basics: mammogram vs. MRI. TIME Magazine, October 15, 2007. Time, Inc., New York, NY.
14 American Cancer Society. Cancer Facts & Figures 2007, page 9. Atlanta: American Cancer Society; 2007.
15 Shen Y, Zelen M. Screening sensitivity and sojourn time from breast cancer early detection trials: mammograms and physical examinations. J Clin Oncol 2001; 19:3490-3499.
16 Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film screen mammography for breast-cancer screening. N Engl J Med 2005; 353:1773-1783.
17 Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75-89.
18 Sardanelli F, Podo F. Breast MR imaging in women at high risk of breast cancer. Is something changing in early breast cancer detection? Eur Radiol 2007; 17:873-887.
19 Warner E, Plewes D, Hill K, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast exam. JAMA 2004; 292:1317-1325.
20 Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004; 351:427-437.
21 Warner E, Plewes D, Hill K, et al. Effect of age and temporal patterns over 5 years in a magnetic resonance imaging-based breast surveillance study for BRCA mutation carriers. Proc Am Soc Clin Oncol 2004; 23:831 (abstr).
22 Bigenwald R, Warner E, Gunasekara K, et al. Is mammography adequate for screening BRCA mutation carriers with low breast density? Proc Am Soc Clin Oncol 2006; 24:544s (abstr).
23 Dent R, Warner E. Screening for hereditary breast cancer. Semin Oncol 2007; 34:392-400.
24 Hukkinen K, Pamilo M. Does computer-aided detection assist in the early detection of breast cancer? Acta Radiol 2005; 46:135-139.
25 Jong RA, Yaffe MJ, Skarpathiotakis M, et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology 2003; 228:842-850.
26 Chan HP, Wei J, Sahiner B, et al. Computer-aided detection system for breast masses on digital tomosynthesis mammograms: preliminary experience. Radiology 2005; 237:1075-1080.
27 Tabar L, Fagerberg G, Duffy SW, Day NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J Epidemiol Community Health 1989; 43:107-114.
28 Rosenquist CJ, Lindfors KK. Screening mammography beginning at age 40 years: a reappraisal of cost-effectiveness. Cancer 1998; 82:2235-2240.
29 Lindfors KK, McGahan MC, Rosenquist CJ, Hurlock GS. Computer-aided detection of breast cancer: a cost-effectiveness study. Radiology 2006; 239:710-717.
30 Blanchard K, Colbert JA, Puri D, et al. Mammographic screening: patterns of use and estimated impact on breast carcinoma survival. Cancer 2004; 101:495-507.
31 Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA 1/2 mutation carriers with breast magnetic resonance imaging. JAMA 2006; 295:2374-2384.
32 Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003:72:1117-1130.
33 Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005; 23:8469-8476.
34 Hollingsworth AB, Stough RG. Breast MRI screening for high-risk patients. Sem Breast Dis (accepted for publication in 2008).
35 ACR BI-RADS®-Mammography, 4th Edition. In: ACR Breast Imaging Reporting and Data System, Breast Imaging Atlas. Reston, VA. American College of Radiology; 2003.
36 Duffy M. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem 2006; 52:345-351.
37 Bolan PJ, Nelson MT, Yee D, Garwood M. Imaging in breast cancer: magnetic resonance spectroscopy. Breast Cancer Res 2005; 7:149-152.
38 Hofmann M. From scinti-mammography and metabolic imaging to receptor targeted PET – new principles of breast cancer detection. Phys Med 2006; 21 Suppl 1:11.
 For an individual with either type-1 or type-2 diabetes, the threat of hypoglycemia — commonly known as low blood glucose — is constant. Blood-sugar levels that drop too low present a variety of health risks, and exercise can cause blood-glucose levels to drop in both diabetics and those without diabetes. When exercising, it’s important to understand the relationship between physical activity, food intake and hypoglycemia.
For an individual with either type-1 or type-2 diabetes, the threat of hypoglycemia — commonly known as low blood glucose — is constant. Blood-sugar levels that drop too low present a variety of health risks, and exercise can cause blood-glucose levels to drop in both diabetics and those without diabetes. When exercising, it’s important to understand the relationship between physical activity, food intake and hypoglycemia.

 Worried about sports injuries? Don’t sweat it. Think of avoiding injury as just another part of playing by the rules only this rulebook is the one that keeps you from getting hurt. That’s because the best way to deal with sports injuries is to prevent them. Prevention includes knowing the rules of the game you’re playing, using the proper equipment, and playing it safe.
Worried about sports injuries? Don’t sweat it. Think of avoiding injury as just another part of playing by the rules only this rulebook is the one that keeps you from getting hurt. That’s because the best way to deal with sports injuries is to prevent them. Prevention includes knowing the rules of the game you’re playing, using the proper equipment, and playing it safe.
 Foot Injuries
Foot Injuries




 Generalized anxiety disorder (or GAD) is characterized by excessive, exaggerated anxiety and worry about everyday life events with no obvious reasons for worry. People with symptoms of generalized anxiety disorder tend to always expect disaster and can’t stop worrying about health, money, family, work, or school. In people with GAD, the worry often is unrealistic or out of proportion for the situation. Daily life becomes a constant state of worry, fear, and dread. Eventually, the anxiety so dominates the person’s thinking that it interferes with daily functioning, including work, school, social activities, and relationships.
Generalized anxiety disorder (or GAD) is characterized by excessive, exaggerated anxiety and worry about everyday life events with no obvious reasons for worry. People with symptoms of generalized anxiety disorder tend to always expect disaster and can’t stop worrying about health, money, family, work, or school. In people with GAD, the worry often is unrealistic or out of proportion for the situation. Daily life becomes a constant state of worry, fear, and dread. Eventually, the anxiety so dominates the person’s thinking that it interferes with daily functioning, including work, school, social activities, and relationships.
 Over 1.1 million women are diagnosed worldwide each year with breast cancer,1 and this number has almost doubled since 1975.2 In addition, breast cancer is the most prevalent cancer in the world, with 4.4 million survivors up to 5 years following diagnosis,1 and millions more surviving longer than 5 years.
Over 1.1 million women are diagnosed worldwide each year with breast cancer,1 and this number has almost doubled since 1975.2 In addition, breast cancer is the most prevalent cancer in the world, with 4.4 million survivors up to 5 years following diagnosis,1 and millions more surviving longer than 5 years. STR
STR A report released by the University of Minnesota Boynton Health Service is the first of its kind in the nation to conduct a comprehensive survey on the health of college students. About 10,000 college students completed the survey. Although the study is focused on students from 14 campuses in Minnesota, the health findings here reflect national health trends for college students, says Dr. Ed Ehlinger, the director and chief health officer of the university’s Boynton Health Service.
A report released by the University of Minnesota Boynton Health Service is the first of its kind in the nation to conduct a comprehensive survey on the health of college students. About 10,000 college students completed the survey. Although the study is focused on students from 14 campuses in Minnesota, the health findings here reflect national health trends for college students, says Dr. Ed Ehlinger, the director and chief health officer of the university’s Boynton Health Service. Health experts say that ADHD (attention deficit hyperactivity disorder) is the most common behavioral disorder that starts during childhood. However, it does not only affect children – people of all ages can suffer from ADHD. Psychiatrists say ADHD is a neurobehavioral developmental disorder.
Health experts say that ADHD (attention deficit hyperactivity disorder) is the most common behavioral disorder that starts during childhood. However, it does not only affect children – people of all ages can suffer from ADHD. Psychiatrists say ADHD is a neurobehavioral developmental disorder.
 Changes In Stair Design Could Help Fight Obesity
Changes In Stair Design Could Help Fight Obesity